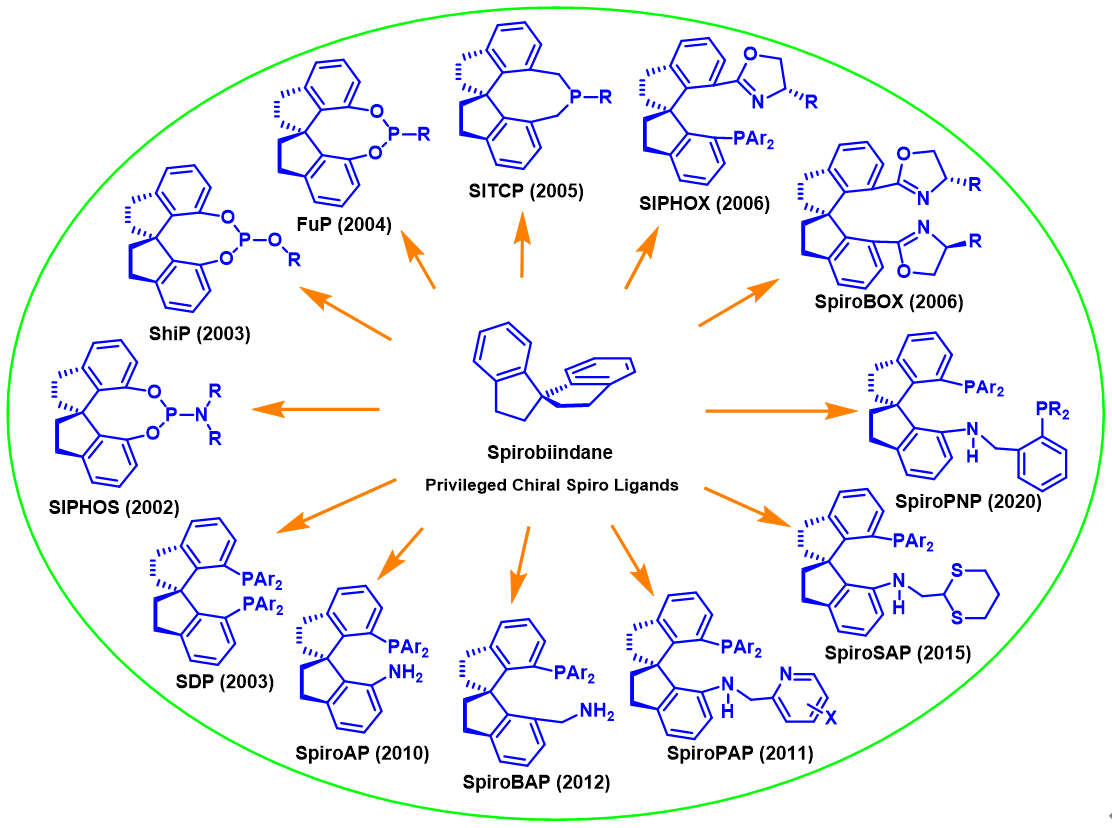
Although a large number of chiral ligands and chiral catalysts have been developed, most of the efficient chiral ligands and chiral catalysts are rooted on a few core structures. Chiral catalysts that show good enantioselectivity in many mechanistically unrelated reactions are called "privileged chiral catalysts". The essence of what makes a catalyst "privileged" is the scaffold (core structure) it possesses. Chiral 1,1'-spirobiindane scaffold collects high rigidity, C2 symmetric, simple chirality, and easy modification and represents an ideal chiral ligand scaffold. Over the past 20 years, starting with the readily available 1,1' -spirodine-7,7' -diol (SPINOL), this lab has synthesized hundreds of chiral spiro ligands including diphosphines SDPs, bisoxazolines SpiroBOXs, amino-phosphines SpiroAP, phosphine-oxazolines SIPHOXs, pyridine-amino-phosphines SpiroPAP, phosphine-amino-phosphines SpiroPNP, and a wide range of monodentate phosphorous ligands SITCPs, ShiPs, FuPs, and SIPHOS, etc. Some of these ligands, such as SIPHOS, ShiPs, SDPs, SIPHOXs, SpiroBOXs, and SpiroPAP are now commercially available from Sigma-Aldrich, Strem Chemicals and Jiuzhou Pharmceutial Co. The chiral spiro ligands have been applied in a variety of reactions, such as asymmetric hydrogenations, carbon–carbon bond formations, and carbon–heteroatom bond formations, and exhibit unique reactivity and enantioselectivity. The chiral spiro ligands have become one of the "privileged" chiral ligands.